Saturated fats (such as butter and lard), we are told, are bad for us, and polyunsaturated fats (such as sunflower oil and corn oil) are good for us. But this is just looking at the fats at room temperature. When they’re heated, they can change completely, breaking down into harmful chemicals. And then the situation is far more complicated.
So which fats and oils are really best to cook with?
Most people never consider the difference between using an oil at room temperature and using it for cooking. But if they do, then you might hear talk of the ‘the smoke point’ of an oil: the temperature at which the oil begins to smoke. The smoke occurs when the non-fat components and the free fatty acids start to break down. Products like extra virgin olive oil contain a great deal of non-fat components, like the anti-oxidants, so it tends to have a lower smoke point. Very refined oils have a higher smoking point.
However, Professor Martin Grootveld from De Montfort University has shown that when we heat oils and fats other, more dangerous, chemical reactions occur which alter the molecular structure of the oils and fats and create new compounds which could be harmful to our health. And this could completely change the advice we should all follow when choosing a cooking oil.
To date, most of the research has taken place in laboratories, but we wanted to find out what happens when we use oils in our everyday cooking. So we set up our very own Trust Me, I’m a Doctor experiment.
The experiment
We gave a group of volunteers a variety of fats and oils to use in their everyday cooking. These included: vegetable oil, cold pressed rapeseed oil, sunflower oil, refined olive oil, extra virgin olive oil, butter and goose fat. Each volunteer was asked to use a selection of oils to cook a variety of different foods using various different cooking methods. This included cooking chips in a deep fryer and roasting vegetables in the oven.
The volunteers then collected the leftover oil which was sent to Leicester School of Pharmacy where the samples were analysed.
Professor Grootveld and his team also ran a parallel experiment where they heated up these same oils and fats to 180C (a standard frying temperature) and took measurements at various time points. No food was cooked in this oil.
All of the samples were then analysed using a technique called ¹H nuclear magnetic resonance (NMR). Where additional confirmation was required, the researchers used ¹H nuclear magnetic resonance spectroscopy (¹H-NMR spectroscopy) which allows individual samples to be compared in detail against other samples or to the whole group and can detect very subtle differences in the chemical composition of the oils.
What we were looking for
Previous research has shown that thermally stressing fats and oils can change their structure; this process is a complex one, known as oxidation, and results in a number of ‘oxidative products’ being produced from the fat or oil. Studies have shown that consuming or even just inhaling these oxidative products have can some negative health effects and they have been linked to an increased risk of heart disease and cancer.
This oxidation process can progress at different rates depending on the factors such as heat, light but most importantly, the oil which is used.
We wanted to know which oils produced the most harmful oxidative products when used in cooking, especially when used in normal kitchen cooking, with food.
The results
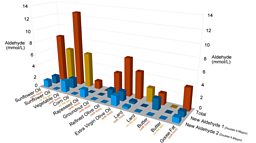
We found that the oils which were rich in polyunsaturated fatty acids, like corn oil and sunflower oil, generated very high levels of oxidative products known as aldehydes. By contrast, the fats and oils which were rich in saturated fatty acids or monounsaturated fatty acids (like butter or olive oil) produced far fewer aldehydes and other potentially dangerous products.
This is because the chemical structure of these oils is very different.
However, interestingly, when analysing the samples our volunteers supplied, Martin identified two new, and potentially dangerous, aldehydes that they had not previously seen in the other oil-heating experiments. These new compounds may have been generated when the oils and fats interact with various foodstuffs. However, Martin and his team intend to analyse these samples further to try and determine the nature of these new compounds.
Why are do some fats produce more toxic products than others when heated?
Saturated fats are so-called because the carbon atoms are “saturated” with four single covalent bonds (they contain no carbon-carbon double bonds). Unsaturated fatty acids contain carbon-carbon double bonds and these make the oil more susceptible to oxidation. Monounsaturated fats (MUFAs) contain one carbon-carbon double bond. And Polyunsaturated fats (PUFAs) contain two or more carbon-carbon double bonds which make them much less stable when they are heated.
So, what oils should I be cooking with?
If you want to try and reduce aldehyde production, Martin suggests that you go for an oil or fat high in monounsaturated or saturated lipids (preferably greater than 60% for one or the other, and more than 80% for the two combined), and low in polyunsaturates (less than 20%). Olive oil is a good compromise because it is about 76% monounsaturates, 14% saturates and only 10% polyunsaturates.
Secondly, when you are cooking try to minimise the amount of oil you use and eat. Soak up excess oil with a paper towel.
Lastly, be careful with how you store your oil. Fats are susceptible to oxidation when they are exposed to light, heat and oxygen so try and keep your oils tightly sealed in a cool, dry cupboard.

